“Rendering of 4D Ultrasound Data with Denoised Monte Carlo Path Tracing” by Petkov, Yu and Houle
Conference:
Type(s):
Title:
- Rendering of 4D Ultrasound Data with Denoised Monte Carlo Path Tracing
Presenter(s)/Author(s):
Entry Number:
- 35
Abstract:
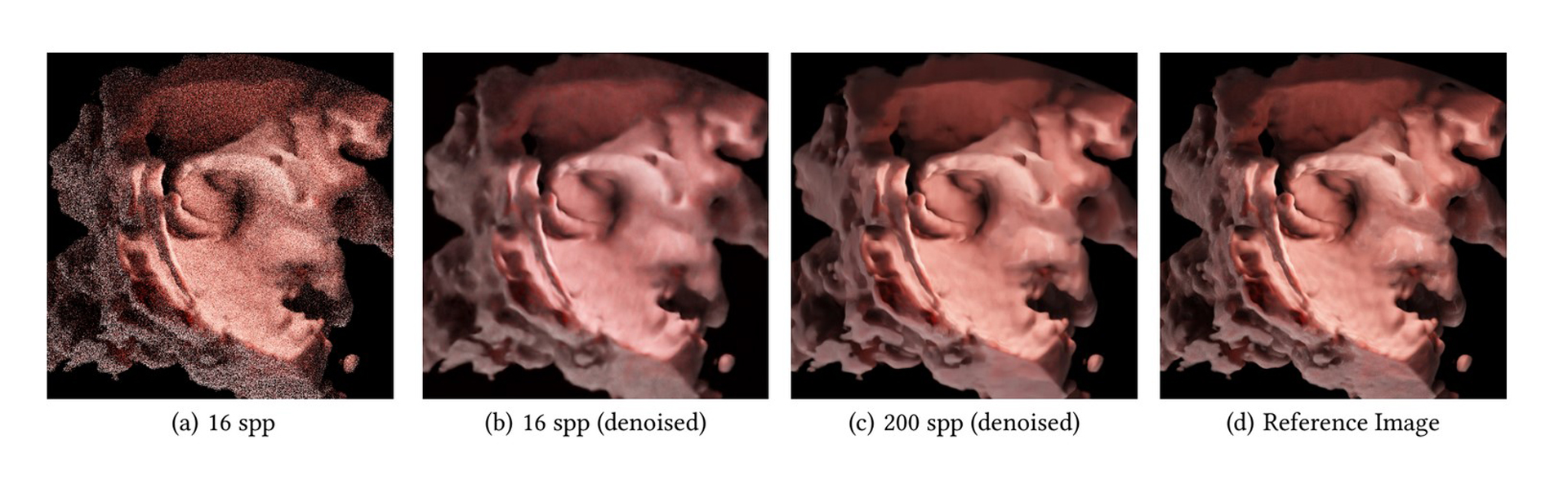
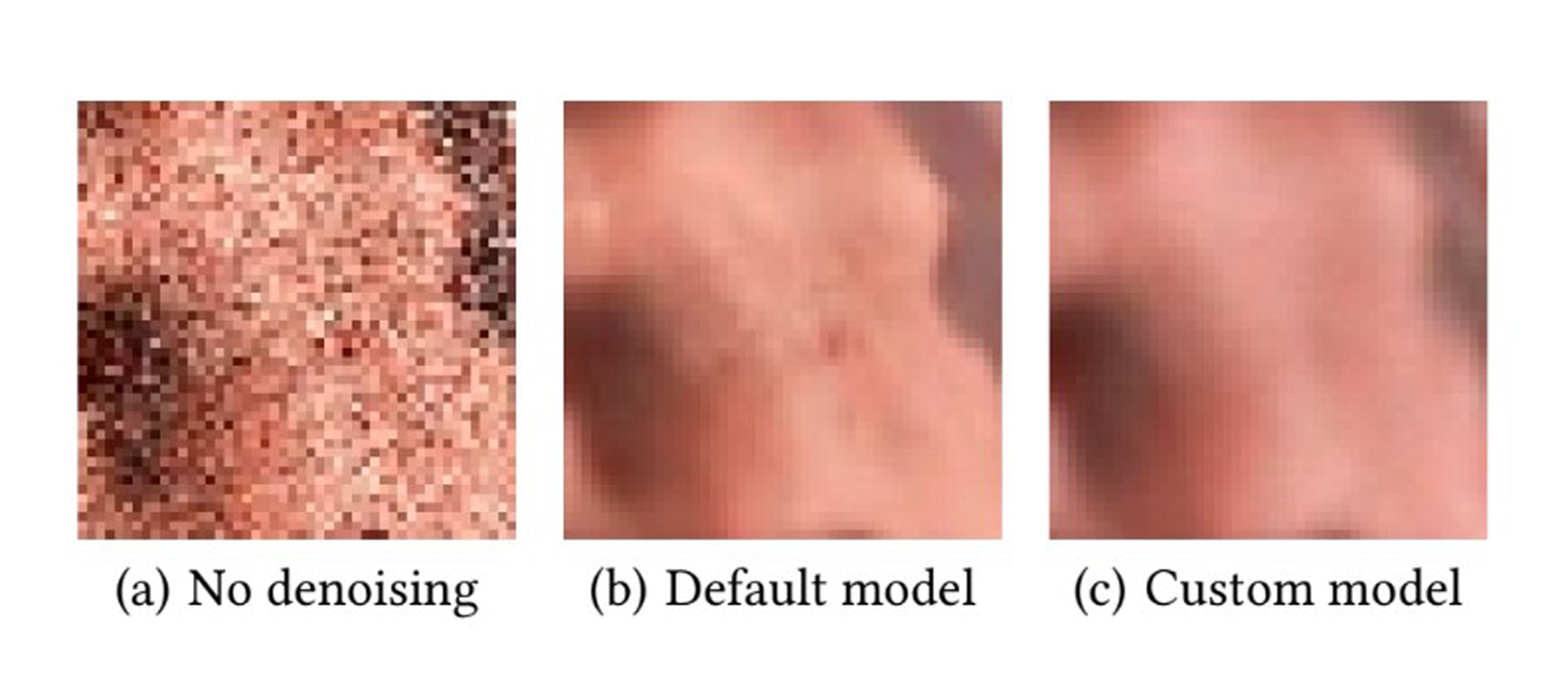
We present a rendering system for 4D ultrasound data based on Monte Carlo path tracing, where a recurrent denoising autoencoder is trained on a large collection of images to produce noise-free images with a reduced number of samples per pixel. While the diagnostic value of photorealistic shading for 3D medical imaging has not been established definitively, the enhanced shape and depth perception allow for a more complete understanding of the data in a variety of scenarios. The dynamic nature of ultrasound data typically limits the global illumination effects that can be rendered interactively, butwe demonstrated that AI-based denoising together with Monte Carlo path tracing can be used both for interactive workflows and for rendering an entire heartbeat sequence at high quality in about a minute, while also allowing for complex lighting environments. Specifically, our contribution is a model compatible with the NVIDIA OptiX interactive denoiser, which has been trained on ultrasound-specific rendering presets and data.
Keyword(s):
Acknowledgements:
- Data courtesy of the Piedmont Heart Institute, Atlanta, GA.
- The authors have a contractual relationship with Siemens Healthineers and have received financial compensation.
- The products/features mentioned in the paper might not be approved in some countries or, for other reasons, not yet commercially available. Furthermore, they might still be under development and not commercially available yet. Due to regulatory reasons their future availability cannot be guaranteed.
- Not all products, services or offers referenced in this paper are approved or offered in every market and approved labeling and instructions may vary from one country to another.
- The paper may include discussions of device features and applications that are not FDA cleared in the United States.